

Pretty sure you can download the maps ahead of time, GPS doesn’t require data, then upload the fixes when you get home.


Pretty sure you can download the maps ahead of time, GPS doesn’t require data, then upload the fixes when you get home.
Do it! it is easy to do at home! Just wear some gloves and safety glasses, those jars can easily shatter during the heating process if you use too hot of a heat source. I recommend a glass top electric stove, or put some kind of metal plate between your jar and the burner to help spread out the heat. Once you seal the jar, take it off the heat right away, so it doesn’t build pressure. I boiled mine for a few minutes before sealing to try and get some of the devolved gasses out, and lightly set the lid on top to help the steam push out all the air.
Yes definitely. The pressure will drop along the vapor pressure curve all the way to the triple point, gently boiling all the way if you remove the heat from the vapor and not directly from the water.
Anything for posterity
Exactly. it was bottled at atmospheric pressure while it was boiling, so 1 atm and 100 degrees C. Check this graph to see the relationship between the water’s temperature and it’s pressure in the jar (since there is no air, only water vapor). If the vapor is condensed, then the pressure drops below the curve on the graph, that is, the pressure in the jar is lowered below the vapor pressure of the water. Any time the pressure is below the vapor pressure, the water will boil, releasing vapor, until the pressure is equal to the vapor pressure. The pressure does not become negative, it is still positive, just lower than the vapor pressure at the given temperature. You can get below the vapor pressure curve by changing the temperature too, which is what we usually do when boiling water at a pressure near 1 atm (760mmHg)
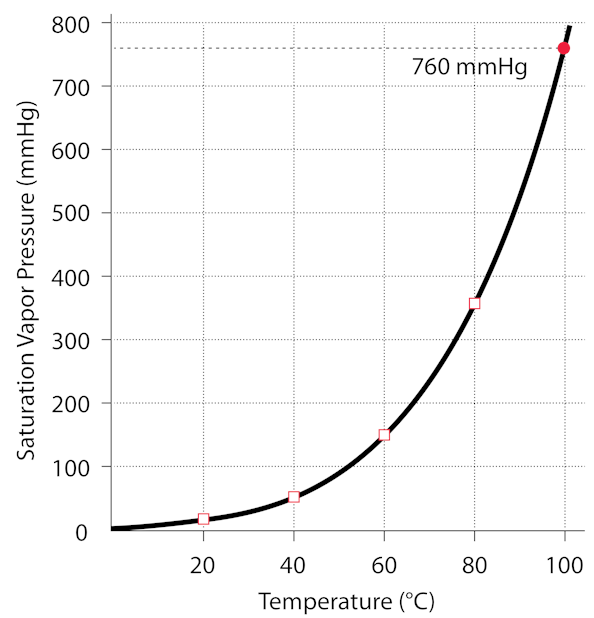
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/watvap.html#c2
(1 atmosphere is ~760mmHg)
a slight aside, there is an important difference between the total pressure of the air, and the partial pressure of water vapor in the air. Inside the jar, the two are equal, but in a dry location (not humid) the partial pressure of water vapor is usually less than the vapor pressure of water at that temperature, but since the total large pressure of the atmosphere would not allow a pocket/bubble of very low pressure water vapor to form inside the bulk water, the water cannot boil, but it will evaporate at the surface anyway until the partial pressure of water is equal to the vapor pressure (very humid).
I decided to translate the worksheet into GLSL code on shadertoy. It was really cool to see the gradients and sub-coordinate systems represented by the intermediate variables in the calculation. Smoke and mirrors. Maybe you might have some insight into some of the calculations. https://www.shadertoy.com/view/cllBzM
Any ideas?
I was thinking of doing three separate GOL simulations, one on each RGB channel, and letting the colors mix that way into like 6 colors. right now, I clamp the pixel brightness values to 0 or 1, so that’s why it’s black/white, or rather black/green.


2nd on the keep notes suggestion. I work on lots of unrelated projects, and each time I end up learning a bunch of new command line utilities, so I try to leave behind a text file describing some of the most useful commands I’d discovered that day. Usually helps me come back to a project and not be back at square one every time.


I thought there was something slightly peculiar about the narration.


The internet is a series of tubes.
I decided to give it a try over the weekend on a road trip, through the apps Organic Maps and Go Map!! I really liked Go Map!! except that it crashes occasionally, and won’t restart until your reinstall it :( loosing all the GPS tracks and unsubmitted data :(( If it was more stable, I’d recommend it to everyone.


Discoverability is something youtube’s alogrithm really gets right, and something lemmy, or the fediverse in general, just sucks at right now.


It’s a really good point. It’s both the algo and the creators the keep us there.


PeerTube is awesome. Also its federated with lemmy!


Time to go to PeerTube. (federated! Bonus)


Might as well go Youtube to PeerTube


I mean, since we’re all here, PeerTube is federated with Lemmy! There are limited numbers of creators on PeerTube right now, but maybe if we can link more videos from there on lemmy and upload some ourselves, we can get the platform into a healthy state. Not that there is nothing there, there is a decent amount uploaded already.
time for some kind of anonymizing location data sharing service, peer to peer or federated protocol? that might be interesting, or sketchy, not sure which.